24th May, 2023 12:00
Fine Instruments of Science, Medicine and Books
Uray, Harold, C, The discovery of the deuteron (deuterium), 1932, and others
1 - The Physical Review, A Journal of Experimental and Theoretical Physics Conducted by the American Physical Society, Vol.39, Second Series, January - March 1932, bound Journal, H. C. Urey, F. G. Brickweed, and G. M Murphy' paper reviewed by Walker Bleakney, 'Additional Evidence for an Isotope of Hydrogen Mass 2' [p536]
2 - Scientific American, vol152, no.6 June 1935, single Journal, Harold, C. Urey's article 'Heavy Water, a new Hydrogen and a New Water' [p.300 - p.302]
3 - Proceedings of the Royal Society of London, vol.219, 7 Oct 1953, bound journal, H. C. Ures's paper titled 'On the Concentration of Certain Elements at the Earths Surface'
4 - Proceedings of the National Academy of Sciences of the United States of America, vol.41 1955Harold C. Ureys paper titled 'On the Origen of Tektites' [p.27 - p.31]
Harold Clayton Urey was an American physical chemist who made significant contributions to the field of isotope chemistry. In 1934, he was awarded the Nobel Prize in Chemistry for his discovery of deuterium, a stable isotope of hydrogen. Urey's groundbreaking work on isotopes laid the foundation for many scientific advances, and his contributions continue to have far-reaching implications to this day.
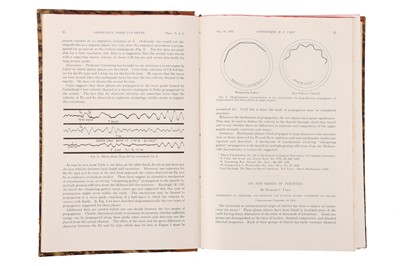
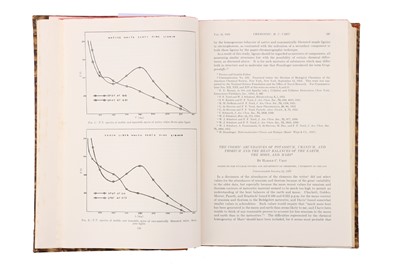
One of Urey's most significant contributions to the study of isotopes was his publication in The Physical Review in 1932, titled "Additional Evidence for an Isotope of Hydrogen of mass 2." In this paper, Urey described his experiments using a mass spectrograph, which allowed him to separate the isotopes of hydrogen based on their masses.
Urey observed that one of the hydrogen isotopes had a mass of 2, which was double the mass of the most common isotope of hydrogen. He hypothesized that this isotope, which he named deuterium, could be used to study the mechanisms of chemical reactions and to trace the movement of atoms in biochemical systems.
Urey's discovery of deuterium opened up new avenues of research in many fields, including nuclear physics, biochemistry, and environmental science. Deuterium is now widely used in a variety of applications, including nuclear reactors, nuclear magnetic resonance imaging (MRI), and the study of global climate change.
In addition to his work on deuterium, Urey also made significant contributions to the study of the isotopes of oxygen, carbon, and nitrogen. His work on isotopes helped to elucidate the origins of the elements in the universe and to establish the age of the Earth.
In conclusion, Harold Clayton Urey was a pioneering physical chemist whose work on isotopes laid the foundation for many scientific advances. His discovery of deuterium, detailed in his article "Additional Evidence for an Isotope of Hydrogen of mass 2" published in The Physical Review in 1932, opened up new avenues of research and has had far-reaching implications for many fields of study. Urey's contributions to science continue to be celebrated today, and his legacy serves as an inspiration to scientists and researchers around the world.
1 - The Physical Review, A Journal of Experimental and Theoretical Physics Conducted by the American Physical Society, Vol.39, Second Series, January - March 1932, bound Journal, H. C. Urey, F. G. Brickweed, and G. M Murphy' paper reviewed by Walker Bleakney, 'Additional Evidence for an Isotope of Hydrogen Mass 2' [p536]
2 - Scientific American, vol152, no.6 June 1935, single Journal, Harold, C. Urey's article 'Heavy Water, a new Hydrogen and a New Water' [p.300 - p.302]
3 - Proceedings of the Royal Society of London, vol.219, 7 Oct 1953, bound journal, H. C. Ures's paper titled 'On the Concentration of Certain Elements at the Earths Surface'
4 - Proceedings of the National Academy of Sciences of the United States of America, vol.41 1955Harold C. Ureys paper titled 'On the Origen of Tektites' [p.27 - p.31]
Harold Clayton Urey was an American physical chemist who made significant contributions to the field of isotope chemistry. In 1934, he was awarded the Nobel Prize in Chemistry for his discovery of deuterium, a stable isotope of hydrogen. Urey's groundbreaking work on isotopes laid the foundation for many scientific advances, and his contributions continue to have far-reaching implications to this day.
One of Urey's most significant contributions to the study of isotopes was his publication in The Physical Review in 1932, titled "Additional Evidence for an Isotope of Hydrogen of mass 2." In this paper, Urey described his experiments using a mass spectrograph, which allowed him to separate the isotopes of hydrogen based on their masses.
Urey observed that one of the hydrogen isotopes had a mass of 2, which was double the mass of the most common isotope of hydrogen. He hypothesized that this isotope, which he named deuterium, could be used to study the mechanisms of chemical reactions and to trace the movement of atoms in biochemical systems.
Urey's discovery of deuterium opened up new avenues of research in many fields, including nuclear physics, biochemistry, and environmental science. Deuterium is now widely used in a variety of applications, including nuclear reactors, nuclear magnetic resonance imaging (MRI), and the study of global climate change.
In addition to his work on deuterium, Urey also made significant contributions to the study of the isotopes of oxygen, carbon, and nitrogen. His work on isotopes helped to elucidate the origins of the elements in the universe and to establish the age of the Earth.
In conclusion, Harold Clayton Urey was a pioneering physical chemist whose work on isotopes laid the foundation for many scientific advances. His discovery of deuterium, detailed in his article "Additional Evidence for an Isotope of Hydrogen of mass 2" published in The Physical Review in 1932, opened up new avenues of research and has had far-reaching implications for many fields of study. Urey's contributions to science continue to be celebrated today, and his legacy serves as an inspiration to scientists and researchers around the world.